Introduction: Equitable participation in clinical trials for all populations is essential to ensure the generalizability of clinical trial results. Historical data suggest that the majority of pediatric patients are enrolled on a trial when treated at a cooperative group institution and minoritized patients are proportionally represented. We proposed to evaluate clinical trial enrollment on Children's Oncology Group (COG) acute lymphoblastic leukemia (ALL) studies to determine if among those who participate in registry studies, there are disparities in participation in therapeutic clinical trials. We hypothesized that minoritized children, those living in disadvantaged neighborhoods, and those living distant from trial sites are less likely to enroll on therapeutic ALL studies.
Methods: This study leverages the Childhood Cancer Research Network (CCRN), a childhood cancer case recruitment protocol that enrolled patients between 2008 and 2015. Within the CCRN registry cohort, we evaluated predictors of therapeutic and non-therapeutic clinical trial enrollment for patients with ALL. Patients >21 years at diagnosis and those without race or ethnicity reported were excluded. Enrollment on an upfront therapeutic trial (that overlapped with the timeframe during which the CCRN protocol was active) was defined as participation on one of the following 10 ALL trials: AALL0232, AALL0331, AALL0433, AALL0434, AALL0631, AALL0932, AALL1131, AALL1231, AALL1331, AALL1621. Enrollment on a non-therapeutic trial was defined as participation in any biology repository (including AALL03B1, AALL05B1, AALL08B1), supportive care, or late effects COG study. Potential predictors of interest included a composite of self-reported race and ethnicity, age at diagnosis, neighborhood socioeconomic status (SES) as measured by the Yost Index at the census tract, and distance to care (calculated as distance from home address to COG hospital). Poisson regression with log link and robust error variance was used to estimate adjusted risk ratios (aRR) and corresponding 95% confidence intervals (CI) for enrollment on a COG therapeutic clinical trial.
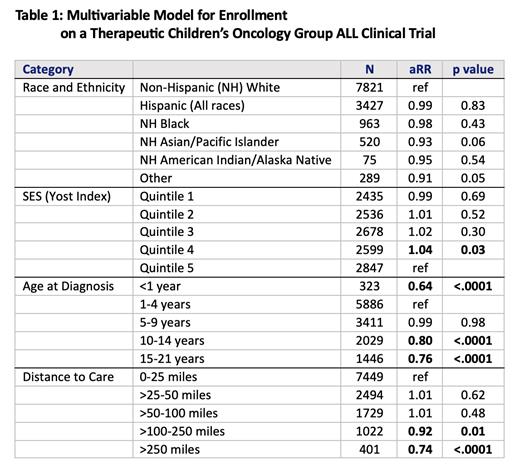
Results: Of the 13,392 patients with ALL enrolled in the CCRN registry, 65% (n=8650) enrolled on a therapeutic COG clinical trial, 9% (1199) enrolled on a non-therapeutic trial without enrolling on a therapeutic trial, and 26% (3543) enrolled in the registry, but no other COG clinical trial. The majority of patient were between 1-9 years old and 58% were non-Hispanic (NH) White, 26% were Hispanic, and 7.2% were NH Black. Almost 11% of patients lived more than 100 miles from the COG center where they received treatment. Neither race nor ethnicity was associated with participation on a clinical trial (as shown in Table 1). Similarly, neighborhood SES was not associated with clinical trial participation. In multivariable models, age and distance to care were both meaningful predictors of therapeutic COG clinical trial enrollment with infants (aRR 0.64, 95%CI 0.57, 0.72) and adolescents (aRR 0.76, 95%CI 0.73, 0.80) less likely to enroll. Those that lived 100-250 miles from their COG center were 8% less likely to enroll (aRR 0.92, 95%CI 0.87, 0.97) and those that lived >250 miles from their COG center were 26% less likely to enroll (aRR 0.74, 95%CI 0.67, 0.81). Age was also a predictor of enrollment on non-therapeutic trials, but distance to care was not associated with enrollment on non-therapeutic trials.
Conclusion: Among those who enroll on COG registry trials (which previous data suggests accounts for 55% of patients with ALL), race, ethnicity, and neighborhood SES are not associated with participation in therapeutic or non-therapeutic clinical trials. While age impacts risk stratification in ALL and thus may have impacted eligibility for various therapeutic trials, greater distance to care emerged as a relevant barrier to enrollment on therapeutic clinical trials. This novel finding in pediatric oncology suggests that interventions focused on ensuring access to clinical trials among those that live more than 100 miles from a COG site should be implemented. Approaches to address this disparity could include allowing study labs to be drawn locally and study visits to be conducted by telehealth when feasible.
Disclosures
Velez:AstraZeneca: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal